Scarabelli Lab Scientific Findings
Endothelial cells undergo apoptosis prior to cardiac cells
We first described the occurrence of apoptosis in different cell types in the isolated Langendorff-perfused rat heart exposed to ischemia/reperfusion injury. Apoptosis was seen mainly in endothelial cells, only after the onset of reperfusion (Figure 3D and Figure 3E). In addition, using a three-step immunocytochemical technique, we showed that the first cells to appear as apoptotic in the very early stage of reperfusion were those in the coronary vasculature, followed, in a later phase of reperfusion, by cardiac myocytes (Figure 3F). Furthermore, the number of positive myocytes decreased with increasing distance from the positive vessel, suggesting that cells of the coronary vasculature may actively contribute to the death of myocytes by releasing pro-apoptotic mediators (Scarabelli TM, et al. Apoptosis of endothelial cells precedes myocyte cell apoptosis in ischaemia/reperfusion injury. Circulation 104(3):253-6, 2001).
Different apoptotic pathways lead to cell death endothelial and cardiac cells
The apoptotic cascade can be initiated either by mitochondrial damage and activation of caspase-9, or by death receptor ligation, which leads to activation of caspase-8. Specific inhibitors of caspase-8 and caspase-9 were used in order to estimate the relative contribution of the two main apoptotic initiating pathways in inducing apoptosis both in endothelial cells and cardiac myocytes in the two phases of ischemia reperfusion. Cleavage of caspase-9 was observed primarily in endothelial cells. Conversely, caspase-8 cleavage was only found in cardiomyocytes, where it progressively rose throughout reperfusion. Consistent with this finding, addition of a specific caspase-9 inhibitor to the perfusate before ischemia prevented endothelial apoptosis, whilst pre-ischemic infusion of a specific Caspase-8 inhibitor affected only myocyte apoptosis (Scarabelli TM et al. Different signaling pathways induce apoptosis in endothelial cells and cardiac myocytes during ischaemia/reperfusion. Circ Res 90(6):745-8, 2002).
Urocortin: the endogenous peptide of cardioprotection
Other experiments were carried out de vivo in order to investigate the hemodynamic, bioenergetic and cytoprotective effects of Urocortin, a 40 amino acid member of the Corticotropin Releasing Hormone family, highly expressed in the cardiovascular system. This endogenous cardiac peptide was administered pre-ischemia, pre-ischemia and during reperfusion, and during reperfusion only, to isolated perfused rat hearts exposed to ischemia. Significant, and in some treatment groups, complete recovery of end diastolic pressure and developed pressure was observed, together with reduction in endothelial and myocyte cell death. In the groups receiving urocortin, significant recovery of high-energy phosphate reservoirs was also seen. Since the cytoprotective and functional benefits are still produced when urocortin is given only at reperfusion, our data suggest that urocortin may be useful clinically in the management of myocardial infarction, especially when associated with a reduction of the ejection fraction (Scarabelli TM et al. Urocortin promotes hemodynamic and bioenergetic recovery and improves cell survival in the isolated rat heart exposed to ischemia/reperfusion. J Am Coll Cardiol 40(1):155-61, 2002).
Cardiac release of urocortin as a potential new marker of sublethal myocardial ischemia
The evaluation of patients with chest pain is often challenging and may be misleading. In patients with a short-lasting heart attack, for instance, cardiac ischemia can be too short in order to induce myocyte necrosis, with subsequent release of the intracellular markers conventionally used to diagnose cardiac damage in the clinical setting. These patients are often admitted to the hospital only after the remission of their symptoms, and therefore exhibit at the same time, negative necrosis biomarkers and a normal electrocardiogram. These individuals are those at major risk to be misdiagnosed and wrongly dismissed. Since we previously documented that urocortin is overexpressed by cardiac cells exposed to ischemia, we assessed whether myocyte release of urocortin is merely a local event, or whether myocyte-derived urocortin is also released in the blood stream and could therefore act as a circulating biomarker useful in the diagnosis of short-lasting heart attacks. Hence, we assayed urocortin in the perfusate of Langendorff-perfused Sprague Dawley rat hearts exposed to increasing periods of zero-flow global ischemia followed by reperfusion, as well as in the circulation of Sprague Dawley rats exposed in vivo to identical periods of regional ischemia and reperfusion. Urocortin levels rose after 5-minutes ischemia and peaked after 10-minutes ischemia, when cell death was not detected. However, myocyte apoptosis and/or necrosis occurred following 20- and 30-minute ischemia, which paralleled a fall in urocortin levels, suggesting that urocortin expression and release are mainly sustained by metabolically challenged, though still viable myocytes (Figure 3G). Therefore, since cardiac release of urocortin, unlike that of conventional biomarkers, occurs before and independently from cell death, urocortin levels may be clinically useful in the diagnosis of sublethal myocardial ischemia due to short-lasting heart attacks. Based on these premises, a clinical trial, enrolling patients admitted to the ER with cardiac chest pain of unknown origin, has been recently started. The goal of this clinical trial will be to probe whether the assessment of urocortin levels is clinically valuable in the differential diagnosis of cardiac chest pain (Knight R. et al. Cardiac release of urocortin precedes the occurrence of irreversible myocardial damage in the rat heart exposed to ischemia/reperfusion injury. FEBS Lett. 19;582(6):984-90, 2008.
Iatrogenic ischemia/reperfusion injury in the human heart
During cardiopulmonary bypass, the human heart is stopped by infusion of a cardioplegic solution, in order to allow and facilitate surgical manipulations. This cardioplegic arrest and subsequent reperfusion inevitably expose the human heart to an iatrogenic ischemia/reperfusion injury. This background encouraged us to evaluate the occurrence of apoptosis and the relative contribution of its signalling pathways in human myocytes from patients exposed to cardiopulmonary bypass, warm blood cardioplegia, and subsequent reperfusion. Furthermore, we investigated whether this mild form of surgical ischemia/reperfusion injury modifies the expression of urocortin in the heart, and, if so, the potential involvement of urocortin as a salvage mechanism. Our study showed for the first time that warm blood cardioplegia induces apoptotic cell death in cardiac myocytes. This myocyte apoptosis, which was shown to involve co-localisation between TUNEL and caspase-3 positive staining, appears to be mainly sustained by the mitochondrial caspase-9-mediated pathway. We demonstrated, moreover, that urocortin expression is increased only in those myocytes, which are not apoptotic, suggesting that endogenous urocortin can also protect the human myocardium from ischemia (Scarabelli TM et al. Warm blood cardioplegic arrest induces mitochondrial-mediated cardiomyocyte apoptosis associated with increased urocortin expression in viable cells. J Thorac Cardiov Surg. 128(3):364-71, 2004).
Minocycline as a new cardioprotective agent
We also investigated the cardioprotective effects of minocycline in primary cultures of both neonatal and adult cardiac myocytes, as well as in the intact heart. Minocycline is a second-generation tetracycline with proven safety that is used in humans for the treatment of minor infections, such as acne and urethritis. Our report showed that minocycline significantly reduced the post-ischemic occurrence of necrotic and apoptotic cell death, with normalization of developed and diastolic pressure. In regard to its antiapoptotic mechanism of action, we observed that minocycline reduced the expression level of initiator caspases, increased the ratio of XIAP to Smac/DIABLO at both the mRNA and protein level, and prevented the mitochondria-mediated release of cytochrome c and Smac/DIABLO. These synergistic actions dramatically reduced the post-ischemic induction of caspase activity associated with cardiac ischemia/reperfusion injury. Owing to its safety record and multiple novel mechanisms of action, minocycline may be a valuable cardioprotective agent to ameliorate the cardiac dysfunction and cell loss associated with acute and chronic ischemia reperfusion injury (Scarabelli TM et al. Minocycline inhibits caspase activation and reactivation, increases the ratio of Xiap to Smac/Diablo and reduces the mitochondrial leakage of cytochrome c and smac/Diablo. J Am Coll Cardiol. 43(5):865-74, 2004)
Oral supplementation with mixed essential amino acids
Amino acid and cardioprotection
In patients undergoing coronary operations, one mechanism of cardiac adaptation to the iatrogenic ischemia/reperfusion injury associated with cardioplegic arrest, is the uptake of amino acids (AA), which was shown to correlate with oxygen consumption. Based on these premises, we investigated whether oral supplementation with mixed AA may protect the rat heart exposed to ischemia/reperfusion injury. Our study documented that long-term oral supplementation with mixed L-amino acids (AA) attenuated the extent of both necrotic and apoptotic cell death following ischemia/reperfusion injury, and induced a significant post-ischemic recovery of cardiac function. This cardioprotective action was achieved, at least in part, through preservation of the energy-producing properties of mitochondria. More specifically, we showed that ATP content and rate of ATP production in isolated mitochondria were reduced by over 75% in ischemic/reperfused control hearts after 2 hours of reperfusion, and returned toward values of non-ischemic control group in hearts supplemented with AA before undergoing ischemia/reperfusion (Scarabelli TM et al. Nutritional supplementation with mixed essential amino acids enhances myocyte survival preserving mitochondrial functional capacity during ischemia/reperfusion injury. Am J Cardiol. 93(8A):35A-40A, 2004).
Amino acid and sarcopenia
Sarcopenia is an inevitable age-related degenerative process chiefly characterized by decreased synthesis of muscle proteins and impaired mitochondrial function, leading to progressive muscle mass loss. Combinations of aerobic, resistance, and stretching exercise programs exert well-known beneficial effects on minimizing the progression of sarcopenia. However, despite growing evidence of physical and psychological benefits provided by exercise training in the elderly, sedentary behavior increases with age, mainly because the perception of physical frailty associated with sarcopenia made substantial proportions of the older adults basically inactive. Since we previously showed that essential amino acids (AA) can protect cardiac mitochondrial function against ischemia-reperfusion injury, by acting on the energy-producing ability of mitochondria, we sought to probe whether long-term administration of oral AA can also increase protein and ATP content in the gastrocnemius muscle of aged rats, enhancing functional performance. To this end, 6 and 24 mo-old male Fisher 344 rats were divided into 3 groups:group A (6 mo-old rats) and group B (24 mo-old rats) were used as adult and senescent control group respectively, while group C (24 mo-old rats) was used as senescent treated group and underwent 1-month oral treatment with a mixture of mainly essential AA. Untreated senescent animals exhibited a 30% reduction in total and fractional protein content, as well as a 50% reduction in ATP content and production versus adult control rats. Long-term supplementation with mixed AA significantly improved protein and high-energy phosphates content, as well as rate of mitochondrial ATP production, conforming their values to those of adult control animals. The improved availability of protein and high-energy substrates in the gastrocnemius muscle of treated aged rats paralleled a significant enhancement in functional performance assessed by swim test, with dramatic elongation of maximal exertion times versus untreated senescent rats. In line with these findings, we observed that, after 6 hours of rest following exhaustive swimming, the recovery in mitochondrial ATP content was ~70% in adult control rats, ~60% in senescent control rats and normalized in treated rats, as compared to animals of the same age unexposed to maximal exertion. In conclusion, nutritional supplementation with oral AA improved protein and energy profiles in the gastrocnemius of treated rats, enhancing functional performance and accelerating high-energy phosphates recovery following exhaustive exertion. These findings, taken together, would suggest that oral intake of mixed essential AA by the elderly population can be used as a therapeutic remedy to slacken, or even reverse, the progressive anatomical and functional skeletal muscle degeneration associated with sarcopenia. A clinical trial aimed at testing the scientific soundness of this intriguing hypothesis will be started in the next few months (Chen-Scarabelli C et al. Oral administration of amino acidic supplements improves protein and energy profiles in skeletal muscle of aged rats: Elongation of functional performance and acceleration of mitochondrial recovery in adenosine triphosphate after exhaustive exertion. Am J Cardiol. June 2008).
Amino acid and mechanism of action
Since we have previously demonstrated that the transcription factor STAT1 plays a critical role in promoting apoptotic cell death, whereas the related STAT3 family member may antagonize STAT1 and protect cardiac myocytes from ischemia/reperfusion injury, we investigated whether short-term nutritional supplementation with the same mixture of amino acids (AA) has any effects on STAT1 and/or STAT3 activation in the Langendorff perfused rat heart exposed to ischemia/reperfusion injury. In Sprague-Dawley rats given a single oral dose of essential AA (1 g/kg), and exposed, after 6 hours, to 35 minutes of ischemia, followed by 120 minutes of reperfusion, AA supplementation prolonged STAT3 activation/phosphorylation, while STAT1 activation was significantly reduced. Enhanced STAT3 phosphorylation paralleled a reduction in expression of Fas, a known STAT1 target gene and pro-apoptotic marker that is upregulated after ischemia/reperfusion. Moreover, abrogation of STAT3 activation by means of the JAK inhibitor AG490, reduced, but did not abolish, the cardioprotective effects of AA supplementation after ischemia/reperfusion injury. These results show that modulation of the functional balance between STAT3 and STAT1, with preferential activation of pro-survival STAT3 over the pro-apoptotic STAT1, represents one mechanism by means of which short-term oral supplementation with mixed AA protects the heart from ischemia/reperfusion injury (Scarabelli TM et al. Amino acid supplementation differentially modulates STAT-1 and STAT-3 activation oin the myocardium exposed to ischemia/reperfusion injury. Am J Cardiol. June 2008.)
Ephedra intoxication in the human heart
Ephedra, also known as "ma huang, ephedra sinica, ephedrine, sida cordifolia, and epitonin", has been used in China for thousands of years to treat asthma and other respiratory disorders. This herb has been reported to stimulate the sympathetic nervous system and the heart, and suppress appetite. The reported effects have resulted in wide marketing of this substance in dietary supplements for weight loss, as well as for enhanced athletic performance. However, supplements containing ephedra or its alkaloid derivative, ephedrine, have been linked to serious adverse events, including seizure, stroke, and cardiovascular effects, ranging from hypertension and myocardial infarction to sudden death. We recently described the case of a 45-year-old woman who died of cardiovascular collapse while taking Ephedra. Tissue analysis revealed non-specific degenerative alterations in the myocardium (lipofuscin accumulation and basophilic degeneration and vacuolation of myocytes, Figure H), associated with myocyte apoptosis, caspase activation, and extensive cleavage of miofibrillary proteins a-actin, a-actinin, and cardiac troponin T. Healthcare professionals are therefore urged to warn their patients about the risk of serious adverse effects, which may follow ephedra intake (Chen-Scarabelli C et al. A case of fatal ephedra intake associated with lipofuscin accumulation, caspase activation and cleavage of myofibrillary proteins. Eur J Heart Fail. 7(5):927-30; 2005).
LONG-STANDING NATIONAL AND INTERNATIONAL COLLABORATORS:
Roy McCauley, Ph.D. - Professor, Department of Pharmacology, Wayne State University.
Louis Saravolatz, M.D. - Professor and Chief of Internal Medicine, St John Hospital
David Latchman, Ph.D.- Professor, Master of Birkbeck, University of London, England, UK.
Richard Knight, M.D., Ph.D.- Associate Editor, Cell Death and Differentiation, Nature Group.
Anastasis Stephanou, Ph.D. - Reader, Director, Medical Molecular Biology Unit, Institute of Child Health, University College London, England, UK.
Paul Townsend, Ph.D. - Reader, Division of Human Genetics, University of Southampton, England, UK.
Giuseppe Faggian, M.D. - Professor, Chief, Division of Cardiac Surgery, University of Verona, Italy.
Alessandro Mazzucco, M.D. - Professor, Master of University of Verona, Italy.

CENTER FOR HEART AND VESSEL PRECLINICAL STUDIES
The basic cardiovascular laboratory, based at St John Hospital, occupies 1200 square feet and offers the latest generation equipment, including a 5-foot vertical culture hood, a chemical hood, two CO2 incubators, electroporator, Olympus inverted fluorescent microscope and stereoscope, Zeiss microscope, two hypoxic chambers (for the simulation of cardiac ischemia in cultured myocyte cells), plate reader, multi-channel Chemi-Imager, SDS PAGE and agarose gel electrophoresis, thermocycler PCR machine, real-time PCR machine, high and low speed refrigerated centrifuges, trans-blot systems and a film developer.
The laboratory is connected to a multi-windowed and comfortable Fellows' Room, equipped with 4 desktop computers and two laptops, wireless internet access (for the use of personal laptops), laser printers, scanners, fax machines and full-access to online journals. The Fellows' Room also hosts a small kitchen area, providing refrigerator, microwave oven, toaster oven and coffee machine.
Adjacent to the laboratory is located an independent Conference Room, where lab meetings and journal clubs are usually held.
| CURRENT LABORATORY PERSONNEL Zhaokan Yuan: Postdoctoral fellow |  |
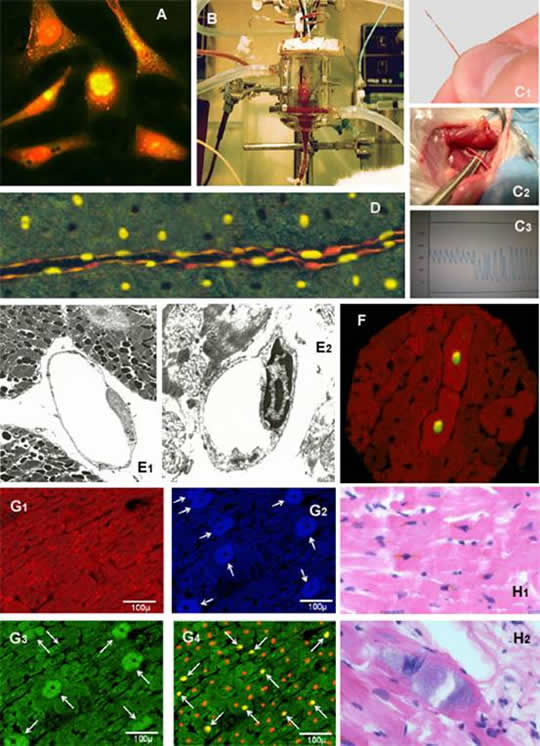 Figure 3A: Primary cultures of neonatal cardiac cells stained with propidium iodide and TUNEL. Two of the six cells, showing yellow nuclear staining, are undergoing apoptotic cell death.
Figure 3A: Primary cultures of neonatal cardiac cells stained with propidium iodide and TUNEL. Two of the six cells, showing yellow nuclear staining, are undergoing apoptotic cell death.
Figure 3B: The isolated Langendorff-perfused rat heart.
Figure 3C: In vivo measurement of cardiac hemodynamics in the rat. A miniaturized 2-Fr Millar catheter (C1) is introduced into the right carotid artery and subsequently advanced into the left ventricle (C2). The correct position of the catheter is evaluated by looking at the shape of the pressure waveform, which has a typical arterial shape when located in the carotid artery and aorta, and acquires a ventricular shape when correctly positioned into the left ventricle (C3).
Figure 3D: TUNEL positivity detected in endothelial cells, specifically labeled by an anti-von Willebrand factor antibody. Figure 3E:Electron microscopy picture of an endothelial cell showing normal nuclear features (E1) and typical hallmarks of apoptotic cell death, namely chromatin margination and condensation (E2).
Figure 3F: Double immuno-staining of a rat myocardial section showing co-localization of TUNEL (yellow nuclear staining) and cleaved active form of caspase-3 (cytosolic positive labeling) in two contiguous cardiac cells.
Figure 3G: Four adjacent myocardial sections serially cut from the same isolated Langendorff-perfused rat heart exposed to 30 minutes ischemia and 2 hours reperfusion. Desmin-positive myocytes exhibiting a typical "red banding". Same myocyte population, stained by fluorescent in situ hybridization (G2) and immunohistochemistry (G3), showing expression (white arrows) of urocortin mRNA and protein, respectively. Matching myocyte population, counterstained with propidium iodide (orange nuclei), showing nuclear TUNEL-positive staining (yellow nuclei pointed by white arrows). Importantly, myocyte showing cytosolic positive staining for urocortin mRNA and protein are systematically TUNEL negative.
Figure 3H: Haematoxylin and eosin section of myocardium from a 45-year-old woman, who died of cardiovascular collapse while taking ephedra. Myocytes containing lipofuscin pigment as brown granules with a predominantly perinuclear distribution (H1). Basophilic degeneration of a cardiac myocyte exhibiting a blue/grey amorphous material within the cytoplasm (H2).